Principal investigators are often asked by their patients if they can still access the investigational product (IP) after they have completed the clinical trial. In some medical device trials, using an implantable product for example, typically the product is provided to the participant during the trial, and not required to be returned.
In pharmaceutical and some medical device trials, access to IP following the end of a trial may be a desired outcome for the patient, but not planned for within the clinical trial protocol by the sponsor. However, supply of the product may still be possible through these pathways, which are also outlined on the Australian Government Therapeutic Goods Administration (TGA) https://www.tga.gov.au/accessing-unapproved-products
1. Extension Clinical Trial
The sponsor may choose to create an extension trial, such that participants consent to roll into the extension trial upon completion of the initial trial. The benefits to the sponsor in this situation is collection of long-term safety and efficacy data. The extension trial, like all clinical trials, requires ethics approval and the TGA is notified through the Clinical Trial Notification (CTN) or Clinical Trial Exemption (CTX) Scheme.
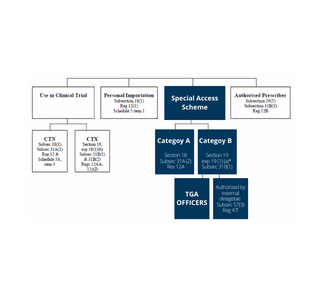
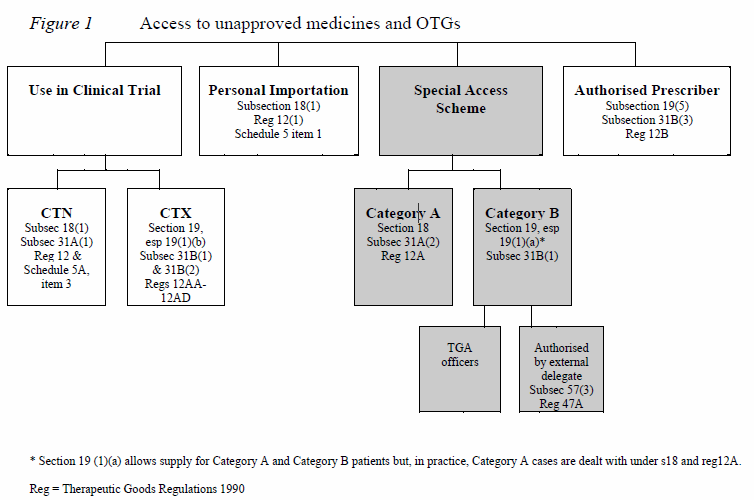
2. Special Access Scheme (SAS)
The SAS is an arrangement which provides for the import and/or supply of an unapproved therapeutic good for a single patient, on a case by case basis. There are two categories under the SAS:
Category A – A patient who is seriously ill with a condition from which death is reasonably likely to occur within a matter of months, or from which premature death is reasonably likely to occur in the absence of early treatment.
Category B – All other patients.
3. Authorised Prescribers
The authorised prescriber an arrangement with the TGA, where the medical practitioner is granted authority to become an authorised prescriber of a specific unapproved product to specific patients who have a specific medical condition. The physician must have expertise in the medical condition being treated, be able to determine the needs of the patient, and commit to monitor the outcome of the therapy.
The authorised prescriber TGA application – agreement to treatment directions authorisation of prescribers under section 19(5), 32CM or section 41HC of the Therapeutic Goods Act 1989; must include an endorsement from an ethics committee.
As an authorised prescriber must complete six-monthly reports to the TGA outlining patient numbers, dose, duration, and any related adverse events.
Under the SAS and Authorised Prescriber, conditions imposed may include:
Restrictions on the maximum dose and duration of treatment; treatment not be discontinued prior to the end of a treatment period; unapproved therapy be regarded as experimental; the doctor and patient, or guardian, accept responsibility for any adverse events; upon completion all remaining supplies are returned to the supplier.
Additionally, the medical practitioner is responsible to liaise with the supplier in order to provide the IP. Arrangements should be made for delivery to a doctor or pharmacy to allow for labelling and any additional instructions. A prescription is required for dispensing of medicinal products, which requires the TGA approval number.
4. Personal Import Scheme
Personal importation is where an individual either brings a therapeutic good into Australia on their person or arranges from within Australia for a therapeutic good to be sent to them from an overseas supplier. The criterion is goods must be used by that individual or a member of his/her immediate family, and are not sold or supplied to any other person. In this situation the individual assumes responsibility for any adverse events of the unapproved product.